Use of synthetic zeolites for arsenate removal from pollutant water
Abstract
Arsenic is known to be a hazardous contaminant in drinking water that causes arsenical dermatitis and skin cancer. In the present work, the potential use of a variety of synthetic zeolites for removal of arsenic from water below the current and proposed EPA MCL has been examined at room temperature. Experiments have been conducted to examine the extent of arsenic removal as a function of pH. The effect of initial arsenic concentration and liquid to solid ratio in the batch reactions has also been studied. Zeolite NH4 /Y (NY6) showed significant arsenate removal capacity over a wide initial pH range of 2–12. Zeolite NY6 achieved this performance by buffering the initial pH to within a range of 3.5opHo7 where uptake of arsenate onto aluminol surface groups is at a maximum. The high aluminum content of NY6 (i.e. low Si/Al ratio) was an important factor governing the improved performance of this zeolite relative to other tested zeolites with higher Si/Al ratio. The pH buffering capacity of NY6 could lead to savings in cost and process time for industrial effluent treatment due to avoidance of a pH pre-conditioning step prior to arsenate removal.
1. Introduction
The presence of arsenic in water is due to the dissolution of minerals from subterranean strata or from an anthropogenic origin such as the leaching of manmade arsenic compounds from smelting of metal ores, agricultural pesticides, desiccants and wood preservatives. The presence of arsenic in water supplies has been linked to arsenical dermatitis, skin cancer [1], neurological effects, enlargement of liver, heart disease and internal cancers [2]. Several techniques have been developed to remove arsenic from water. Adsorption [3,4], anion exchange [5], reverse osmosis [6] and coagulation processes [7,8] are some of the commonly used techniques. Alumina [3] and hydrous iron oxides [8] are commonly employed for arsenic removal in adsorption and coagulation methods.
Zeolites are well known for their ion exchange capacity [9–11]. The role of zeolites in the conversion of solid and liquid hazardous wastes into environmentally acceptable products has also been demonstrated [12,13]. Several zeolites, namely clinoptilolite [14], chabazaite [14], SZP1 [15,16], 13X [17] and 5A [17] have been identified as potential candidates for arsenic removal from water.
Synthetic zeolites are useful because of their controlled and known physico-chemical properties relative to that for natural zeolites. The focus of the present study was evaluation of the effectiveness of the synthetic zeolites Y, Ferrierite, ZSM-5 and Beta in their H+ and NH4 forms for removal of arsenate from water over a wide range of pH. In published reports on arsenic removal using activated alumina, adsorption via ligand exchange has been identified as the removal mechanism [3,18]. A similar reaction is possible for adsorption of arsenic onto zeolites, where terminal aluminol or silanol hydroxyl groups develop at the edges of the zeolite particle. This phenomenon has been observed for phyllosilicate minerals where terminal aluminol or silanol groups participate in arsenic adsorption reactions [19]. It has been observed that the H+ and NH4 forms of the synthetic zeolites listed above were capable of removing arsenate to o50 ppb within 15 min [20], which is the current permitted maximum contaminant level (MCL) for arsenic in the United States [21]. Special attention was given to the ability of the zeolite to remove arsenic to a concentration below 10 ppb, which will be the effective MCL from year 2006 as established by the US Environmental Protection Agency [21].
2. Experimental
Different commercial zeolite samples (Y, ZSM-5, BETA, Ferrierite) were procured in their and H+ + NH4 form (ZEOLYST International, USA). The names, abbreviations, and the structural Si:Al ratio in the synthetic zeolites are shown in Table 1. Arsenic solutions were prepared by dissolving Na2HAsO4 7H2O (J.T. Baker) into deionized water (Millipore 18 MO).
2.1. Reactions
In a typical batch reaction, an aqueous solution (20 ml) containing dissolved arsenate (B5 ppm As) was mixed with the zeolite sample (2 g) in a bottle and kept on a shaker at room temperature. An initial screening study was conducted to examine performance of all zeolites at a fixed total As concentration, pH and liquid:solid ratio for a period of up to 3 h. Subsequent detailed studies were carried out with zeolite NY6 to examine the influence of pH, total As concentration, and liquid:solid ratio. A fixed reaction period of 30 min was employed for studies examining pH and liquid:solid ratio effects, since the screening study indicated that steady state was achieved within this period of time. After completing the reaction, suspensions were centrifuged (5 min, 3600 rpm, Beckman CS-6KR Centrifuge) and filtered (0.2 mm nylon syringe filter) to achieve solid– liquid separation. The pH of the solution was monitored prior to and following reaction with the zeolite.
Table 1 Si/Al ratio, channel dimensions, and manufacturer reported surface area of the synthetic zeolites tested in this research
| Zeolite | Abbreviation | Si/Al | Channel dimensions | Surface area (m2/g) |
|---|---|---|---|---|
| NH4+/Ferrierite | NFER | 27.5 | 10 membered rings (4.3×5.5A Å ) 8 membered rings (3.4×4.8A Å ) |
400 |
| NH4+/ZSM-5 | NZ | 40 | 10 membered rings (5.1×5.5A Å ) |
425 |
| H+/Y | HY40 | 40 | 12 membered rings (7.4×7.4A Å ) |
780 |
| H+/Beta | HB | 37.5 | 12 membered rings (5.6×5.6A Å ) |
NR |
| NH4+/Y | NY6 | 6 | 12 membered rings (7.4×7.4A Å ) |
730 |
| H+/Y | HY6 | 6 | 12 membered rings (7.4×7.4A Å ) |
NR |
NR=not reported.
For zeolite regeneration, 0.1 N NaOH (pH=12.5) and 0.1 N HCl (pH=0.8) solutions were used. Following reaction to remove arsenic from solution, the zeolite was isolated from solution and re-suspended for 15 h in either the acid or base solutions. The regenerated zeolite was subsequently washed, centrifuged and filtered to isolate it from the respective acid or base solution. The zeolite was again treated with an arsenic solution as explained earlier to test for changes to the arsenic removal capacity.
2.2. Analysis and characterization
Chemical analysis of arsenic in sample supernatants was carried out using a Perkin Elmer Optima 3300DV inductively coupled plasma atomic emission spectroscopy (ICP-AES) or a Perkin Elmer 5100ZL graphite furnace atomic absorption spectrophotometer (GFAAS). The arsenic detection limits for ICP-AES and GFAAS were 33 and 2 ppb, respectively. Zeolites were characterized by powder X-ray diffraction (Rigaku
Miniflex) using Cu Ka radiation (30 kV, 15 mA) between 3–60° 2y at a scan speed of 4°2y/min. Changes in pH buffering capacity for unreacted and reacted NY6 zeolite samples were determined via titration with NaOH of suspensions with liquid:solid ratio of 100 ml/ g. Prior to titration, zeolite solids were washed with deionized water via centrifugation until no change in solution specific conductivity was observed (typically o50 mS/cm). Titrations were initiated at the equilibrium pH of the suspension in deionized water prior to base addition up to pH=12.5. The empirical buffer intensity of the suspensions was calculated as a function of pH using the following equation:
Buffer intensity
¼½ðmole NaOHÞ-ðmole NaOHÞd=½pHi -pHi-1d: ð1Þ i i-1
The buffer intensity provides a measure of the capacity of the zeolite to mitigate pH changes during base addition [3].
3. Results and discussion
3.1. Effect of time on different zeolites
Four different zeolites (Y, ZSM-5, Ferrierite and Beta) have been selected for the arsenic removal studies. The purpose for selecting these zeolites was to study the effect of zeolite structure and chemical composition (Si/ Al ratio) on the arsenic removal reaction. The results of the arsenic removal reaction on different zeolites are shown in Fig. 1. The reaction was run for 3 h with an initial arsenic concentration of 5 ppm. H+/Beta (HB), NH4 /Y (NY6) and H+/Y (HY6) were very effective for removal of arsenate. In fact, NH4 /Y (NY6) removed arsenic to a concentration below 50 ppb, which is the current arsenic MCL established by the USEPA. The reaction was completed within 15 min for these zeolites. NH4 /Ferrierite (NFER), NH4 /ZSM-5 (NZ) and H+/Y (HY40) were not effective in the arsenic removal reaction. The different arsenic removal capacity of zeolite Y indicates that the structure, including Si/Al ratio, plays an important role in the arsenic removal process. Such effect of high exchange capacity of high alumina zeolites has been shown previously [9,10]. This may be due to a higher concentration of terminal Al– OH species in low Si/Al ratio zeolites, which leads to a greater capacity for a ligand exchange reaction. An hypothetical ligand exchange reaction can be depicted for H2AsO4as follows:
AlT-OH þH2ASO– AlT-H2ASO4 þOH-: ð2Þ
For this reaction expression, the zeolite terminal aluminol group is identified with the symbol AlT-OH. The reaction stoichiometry will depend on the predominant protonation state of the surface aluminol group and the arsenate oxyanion. The aluminol group is proposed as the reactive site, since the reported adsorption capacity of silanol surface groups in silicon oxide is substantially lower [22,23].
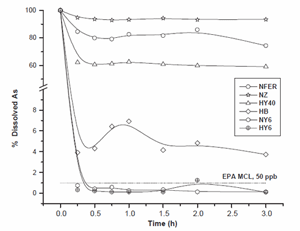
Fig. 1. The removal of arsenate as a function of time on synthetic zeolites Y, ZSM-5, Ferrierite and Beta. Reaction conditions: 5 ppm As; liq/solid=10ml/g; pHB7.5. EPA MCL (50 ppb As) has been plotted based on the 5 ppm initial As concentration.
3.2. Effect of pH
Experiments have been carried out to test the effectiveness of NH4 /Y (NY6) zeolite in arsenic removal over a range of pH (Figs. 2 and 4). The tested initial pH range was 0.76–13.2. X-ray diffraction characterization was carried out before and after the arsenic removal reaction to study the structural changes to the zeolite material due to pH variation (Fig. 3). The initial arsenic concentration of the solution was 5 ppm and the reaction time was 30 min.
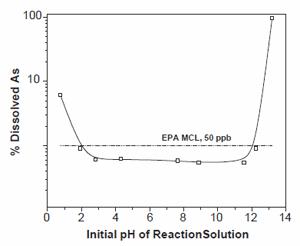
Fig. 2. The effect of initial pH on arsenate removal by synthetic zeolite NH4+/Y (NY6). Reaction conditions: 5 ppm initial As; liq/solid=10 ml/g; 30 min reaction time. EPA MCL (50 ppb As) has been plotted based on the 5 ppm initial As concentration.
The NH4 /Y (NY6) zeolite showed greatest capacity for arsenic removal over the initial pH range 2–12 (Fig. 2). In this pH range the arsenic concentration after the reaction was below detection by ICP-AES (o33 ppb). The structure of the zeolite remained stable over this pH range, which is reflected in the XRD patterns (Fig. 3). However, for an initial pH of 13.2 the zeolite structure was unstable resulting in alteration to predominantly a poorly crystalline aluminosilicate. The solid transformation was accompanied by a nearly two order of magnitude reduction in the efficiency of arsenate removal. Dissolved concentrations of Al and Si showed that less than 1% of the zeolite was solubilized, indicating that loss of arsenate sorption sites due to solid dissolution was not the source of reduced sorption capacity.
A second set of batch sorption experiments was suggest that reduced arsenate sorption is due to competition with aqueous OH -at the final experimental pH (see Eq. (2)). At the lowest initial pH (0.76), the zeolite structure remained intact, but the arsenate removal capacity decreased about 3% from its optimal performance. This reduction in removal capacity occurs over a pH range where arsenate speciation changes from -o predominantly H2AsO4 to H3AsO4 [25]. This behavior has been observed for arsenate sorption onto clay minerals, where sorption begins to decrease below pH 3 [19].
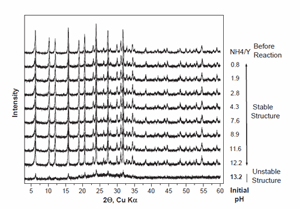
Fig. 3. XRD patterns of the zeolite samples studied in Fig. 2. The initial reaction pH is shown to the right of each pattern to demonstrate the stability of NY6 over a wide pH range.
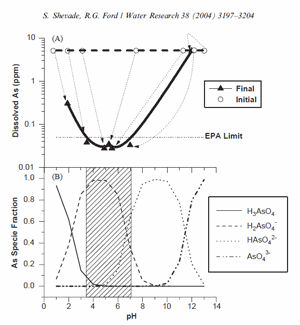
Fig. 4. (A) The effect of pH on arsenate removal by NH4+/Y (NY6). Reaction conditions: 5 ppm initial As; liq/solid=10 ml/g; 30 min reaction time. EPA MCL (50 ppb As) has been plotted based on the 5 ppm initial As concentration. (B) The theoretical distribution of arsenate protonation species as a function of pH. Model results were generated assuming addition of 6.94×10-5 mol Na2HAsO4/l.
In the pH study, it was observed that the zeolite had a significant capacity to buffer highly acidic and alkaline solutions. This was reflected by the buffering of solutions with an initial pH range of 2–12 to within a pH range of 3.5–7.0. The buffered pH range lies within the region where arsenate sorption to aluminol surface groups is a maximum. Titration experiments were conducted with un-reacted (untreated and washed with deionized water) and reacted NY6 zeolite samples in order to assess the pH buffering capacity of the zeolite near the alkaline transition point where removal efficiency decreased by almost two orders of magnitude (see Figs. 2 and 4A). Base addition and buffer intensity of three zeolite samples are shown in Fig. 5A. The unreacted (untreated) zeolite and the zeolite reacted at an initial pH of 12.2 (final pH 7.3) had equilibrated suspension pH in deionized water of approximately 5.570.2 (n ¼ 3) and 7.5, respectively. The sample reacted at an initial pH of 13.2 (final pH 12.6) had an equilibrated suspension pH of approximately 9.970.1 (n ¼ 2).
The initial pH of the equilibrated zeolite suspensions indicates that the zeolite reacted at pH 13.2 has lost the capacity to buffer the suspension to circumneutral pH. In general, the pH of a mineral suspension will tend to equilibrate to the pHzpc of the mineral surface in the absence of a strong aqueous buffer. Changes to protonation–deprotonation characteristics of the mineral surface due to structural transformation will alter the pHzpc and therefore the equilibrium pH of the suspension [24]. All samples demonstrated similar buffer intensity over the pH range of the titration. Comparison of titration results for the un-reacted (untreated) zeolite with results for deionized water and the filtrate from the un-reacted (washed) zeolite indicates that the majority of the suspension buffering capacity can be attributed to the zeolite (Figs. 5B and C). The surface structure of the zeolite reacted at an initial pH of 13.2 has been altered to the extent that its apparent pHzpc is significantly more alkaline than either the unreacted zeolite or the zeolites reacted at pH [12.2]. These results show that surface protonation–dissociation reactions play a significant role in governing the final pH in the sorption experiments. These results corroborate the influence of structural transformation observed for the zeolite above an initial pH of 13 and indicate that preservation of the zeolite structure is critical for adequate pH buffering to achieve efficient arsenate removal under the conditions of these experiments.
Release of Al from the zeolite structure into solution was observed for systems with final pH of 3.48 (36.4 ppm Al) and 12.00 (87.0 ppm Al). Speciation modeling [25] showed that these systems were highly under-saturated with respect to potential precipitate phases such as AlAsO4 . 2H2O, and all systems were highly undersaturated with respect to precipitation of As2O5. These results support the contention that sorption to the zeolite surface controls arsenic removal in these systems.
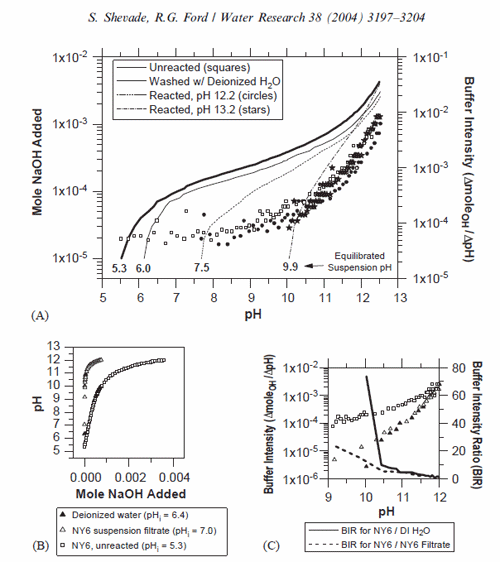
Fig. 5. Titration of NY6 prior to and following reaction at alkaline pH. (A) Base addition (left scale, lines) and calculated buffer intensity (right scale, symbols) are shown as a function of pH. Reaction conditions: liq/solid=100 ml/g, 0.1 or 1N NaOH titrant. (B) Titration of NY6, deionized water, and filtrate from an equilibrated NY6-deionized water suspension. (C) Calculated buffer intensity from titration experiments in Panel B and the ratio of buffer intensities for NY6 relative to deionized water (DI H2O) and NY6 suspension filtrate.
3.3. Effect of initial arsenic concentration
The effect of initial arsenic concentration was studied in different batch reactions on NH4 /Y (NY6) zeolite for a reaction period of 3 h. The initial arsenic concentrations of 100, 50, 5, 1 and 0.5 ppm were evaluated and results are shown in Fig. 6. All the experiments showed decreasing arsenic concentration as a function of time. With initial arsenic concentrations of 1 and 0.5 ppm the concentration was decreased below 2 ppb (0.002 ppm) within 15 min of reaction time. An initial arsenic concentration of 5 ppm was reduced below 33 ppb (0.033 ppm) over the same time period. Greater than 90% removal of arsenic was achieved within 3 h for initial concentrations of 100 and 50 ppm, but arsenic concentrations remained above the target level of 50 ppb.
3.4. Effect of liquid to solid ratio
The effect of liquid to solid ratio in the batch reaction was studied to determine the optimum quantity of solid zeolite required to remove arsenic below the desired level (Fig. 7). Liquid to solid ratios between the ranges of 5–1000 (ml/g) were tested. It was observed that the ratios 5 (ml/g) and 10 (ml/g) were very effective for arsenic removal to a concentration below 50 ppb (0.05 ppm). Arsenic removal was not as effective at higher liquid to solid ratios, which may be due to insufficient capacity for ligand exchange on the NH4 /Y (NY6) zeolite within a 30 min reaction time.
3.5. Regeneration of the zeolites
Preliminary regeneration studies have been carried out on NH4 /Y (NY6) zeolite. In acid regeneration with 0.1 N HCl, only 1% of the sequestered arsenic was recovered. However, the arsenic removal capacity of the same material was unaltered. In base regeneration with 0.1 N NaOH, 30% of the sequestered arsenic was recovered at the cost of almost total loss of arsenic removal capacity of the material. This result is consistent with the observed decrease in zeolite NY6 performance above an initial pH of 13 in batch sorption studies due to induced structural transformation. These results indicate that design of more efficient solutions for zeolite regeneration should avoid basic conditions.
4. Conclusion
NH4 /Y (NY6), /Y (HY6) and H+/Beta (HB) zeolites were very effective for arsenic removal within a very short time (15 min). Results from earlier studies are compared with our detailed results for zeolite NY6 in Table 2. This zeolite was stable over the initial pH range 2–12 and showed very effective arsenic removal over that pH range. The good performance of the NY6 zeolite over the wide initial pH range could be attributed to the pH buffering capacity of this zeolite. In general, zeolites with a lower structural Si:Al ratio demonstrated greater capacity for arsenic removal due to the greater concentration of aluminol surface groups. These results are promising for the use of commercially available synthetic zeolites to treat contaminated ground water and industrial waste effluents, since these materials possess the capacity for cation exchange, anion sorption and acid hydrolysis of organic contaminants. Future work should consider development of tailored zeolites that optimize the capacity for pH buffering while maintaining sorption capacity for contaminants of concern.
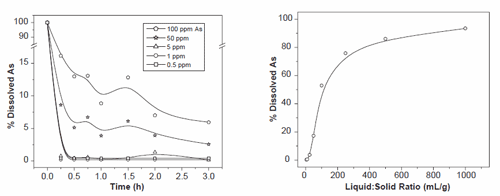
Fig. 6. The effect of initial arsenic concentration on the extent of arsenic removal onto synthetic zeolite NH4+/Y (NY6). Reaction conditions: liq/solid=10ml/g; pHB7.5.
Fig. 7. The effect of liquid to solid ratio on the removal of arsenate onto synthetic zeolite NH4+/Y (NY6). Reaction conditions: 5 ppm initial As; 30 min reaction time; pH=7.5.
Table 2 Comparison of zeolite performance from previous work with results of the current study
| Zeolite | Si/Al | Reaction time (h) | Liq/solid ratio (ml/g) | Initial pH | Final pH | % As removal | Ref. # |
|---|---|---|---|---|---|---|---|
| Fe-clinoptilotite | – | 24.0 | NR | 7.4 | NR | >99.0 | 14 |
| Fe-chabazite | – | 24.0 | NR | 7.4 | NR | >99.0 | 14 |
| Al-SZP | 1.65 | 2.0 | 200 | 3–10.5 | NR | >99.0 | 15, 16 |
| Fe-13X | – | 24.0–48.0 | 60–130 | 4–7 | NR | >99.0 | 17 |
| Fe-5A | – | 24.0–48.0 | 60–130 | 4–7 | NR | >99.0 | 17 |
| Y | 6.0 | <0.25 | 10 | 2–12 | 3.5–7 | >99.0 | Current research |
NR=not reported.
Acknowledgements
The analytical support of Dr. Ning Xu and Ms. Sandra Saye from ManTech Environmental Research Services, Inc. and Justin Thompson from East Central Oklahoma University is acknowledged. The US Environmental Protection Agency through its Office of Research and Development funded and managed the research described here. The work was performed while Dr. Siddhesh Shevade was a National Research Council Research Associate at the Ground Water and Ecosystems Restoration Division. This document has not been subjected to Agency review and therefore does not necessarily reflect the views of the Agency, and no official endorsement should be inferred. The mention of trade names does not imply endorsement.
References
- Dutta A, Chaudhuri M. Removal of arsenic from groundwater by lime softening with powdered coal additive. Aqua 1991;40(1):25.
- Pontius FW. Crafting a new arsenic rule. J Am Water Works Assoc 1994;86(9):6.
- Stumm W, Morgan JJ. Aquatic chemistry: chemical equilibria and rates in natural waters. New York: Wiley; 1996.
- Clifford DA, Ghurye GL. Metal-oxide adsorption, ion exchange and coagulation-microfiltration for arsenic removal from water. In: Frankenberger WT, editors. Environmental chemistry of Arsenic. New York: Marcel Dekker; 2002.
- Ghurye GL, Clifford DL, Tripp AR. Combined nitrate and arsenic removal by ion exchange. J Am Water Works Assoc 1999;91:85–96.
- Sorg TJ, Logsdon GS. Treatment technology to meet the interim primary drinking water regulation for inorganics: Part 2. J Am Water Works Assoc 1978;70:379–92.
- Hering JG, Elimelech M. Arsenic removal by enhanced coagulation and membrane processes. Report 90706. Denver, CO: American Water Works Association and Research Foundation; 1996.
- Driehaus W, Jekal M, Hildebrand U. Granular ferric hydroxide: a novel adsorbent for the removal of arsenic from natural water. J Water SRT, 1998;47:30–5.
- Barrer RM. Hydrothermal chemistry of zeolites. New York: Academic Press; 1982.
- Breck DW. Zeolites molecular sieves. New York: Wiley; 1974.
- Nishino A. Household appliances using catalysis. Catal Today 1991;10(1):107.
- Weitkamp J. in: Weitkamp J, Karge HG, Pfeifer H, Holderich W, editors. Proceedings of the International Symposium on Environmental Catalysis, Tokyo, 1993.
- Iwamoto M. Zeolites in environmental catalysis. Stud Surf Sci Catal 1994;84:1395.
- Bonnin D. Proceedings of the Annual Conference on American Water Works Association, 1997, 421.
- XuY, Ohki A, Maeda S. Adsorption of arsenic(V) by use of alumina-loaded shirasu-zeolite. Chem Lett 1998;10:1015.
- XuY, Ohki A, Maeda S. Removal of arsenate, phosphate and flouride ions by aluminium-loaded shirasu-zeolite. Toxic Env Chem 2000;76(1–2):111.
- Abdel-Fattah M. Tarek, Ansari Z. Ahsan, Voice C. Thomas. Prepr. Ext. Abstr. ACS Natl. Meet., Am Chem Soc, Div Environ Chem 2000;40(1):422.
- Goldberg S, Johnston C. Mechanisms of arsenic adsorption on amorphous oxides evaluated using macroscopic measurements, vibrational spectroscopy, and surface complexation modeling. J Coll Interface Sci 2001; 234(1):204–16.
- Manning BA, Goldberg S. Adsorption and stability of arsenic(III) at the clay mineral-water interface. Environ Sci Technol 1997;31(7):2005–11.
- Shevade S, Ford R, Puls R. Arsenic separation from water using zeolites. Am Chem Soc Div Environ Chem 2001; 41(2): Part 1.
- USEPA. Drinking water standard for arsenic. EPA 815-F00-015, Office of Water, Washington, DC; 2001.
- Wasay SA, Haron MJ, Tokunaga S. Adsorption of flouride, phosphate and arsenate ions on lanthanum impregnated silica gel. Water Environ Res 1996;68(3):295–300.
- Swedlund PJ, Webster JG. Adsorption and polymerization of silicic acid on ferrihydrite and its effect on arsenic adsorption. Water Res 1999;33(16):3413–22.
- Uehara G, Gillman G. The mineralogy, chemistry, and physics of tropical soil with variable charge clays. Boulder, CO: Westview Press; 1981.
- Allison JD, Brown DS, Novo-Gradac KJ. MINTEQA2/ PRODEFA2, A Geochemical Assessment Model for Environmental Systems: Version 3.0 User’s Manual; US Environmental Protection Agency, EPA/600/391/021, 1991.

