The application of natural zeolites for mercury removal: from laboratory tests to industrial scale
Abstract
The paper discusses the application of natural zeolites from clinoptilolite group for mercury removal from industrial effluents from copper smelter and refinery. The experiments included studies on sorption mechanisms (adsorption or ion-exchange) and sorption equilibria. The dominating mechanism of sorption with zeolite was found to be ion exchange and the maximum sorption capacity was evaluated as 1.21 meq/g sorbent. Three cation-exchange groups were identified and deprotonation acidity constants (pKa) 3.1, 7.3 and 10.7 were determined. The results of model experiments were verified by the treatment of the industrial effluent containing elevated levels of Hg2þ ions on the laboratory as well as on full-plant scale. Laboratory and industrial scale experiments showed that zeolite was capable of mercury ions removal from the effluent. Currently, the company uses the proposed method for effluents treatment. © 2004 Elsevier Ltd. All rights reserved.
1. Introduction
The main sources of mercury emissions to land, water and air are the processes of ores mining and smelting (in particular Cu and Zn smelting), burning of fossil fuels (mainly coal), industrial production processes (Hg cell chlor-alkali processes for the production of Cl and caustic soda) and consumption related discharges (including waste incineration) (Haidouti, 1997). It was reported that zeolites are materials that can be used for mercury removal from soils (Haidouti, 1997), flue gases (Morency et al., 2002; Jurng et al., 2002) and solutions (Filippids et al., 1996; Misaelides and Godelitsas, 1995; Zamzow et al., 1990).
Zeolites are considered to be, next to clays, ironoxide-coated sands and activated carbons, low-cost sorbents (Bailey et al., 1999). They are naturally occurring silicate minerals, which can also be produced synthetically. Clinoptilolite is probably the mostly abundant out of more than 40 natural zeolites (Bailey et al., 1999; Ming and Dixon, 1987), making it readily available and inexpensive (Bailey et al., 1999).
Zeolites offer a potential for a variety of industrial uses including molecular sieves, ion-exchangers, adsorbers, catalysts, detergent builders (Haidouti, 1997; Ouki and Kavannagh, 1997; Panayotowa, 2003), the removal of cations from acid mine drainage and industrial waste waters (Mondale et al., 1995). Leppert (1990) reported that zeolites, clinoptilolite in particular, have strong affinity for heavy metal ions. The mechanism of adsorption by zeolites was found to be ion-exchange. In the three dimensional structure there are large channels containing negatively charged sites resulting from Al3þ replacement of Si4þ in the O4 tetrahedral–linked by sharing oxygen atoms in rings and cages-cavities occupied by cations which are weakly held in the structure to compensate the charge imbalance (Bailey et al., 1999; Mondale et al., 1995; Sidheswaran and Bhat, 1997). Zeolites contain various types of cationic sites (Abusafa and Yucel, 2002). The overall negative charge of the anions is balanced by cations that occupy the channels within the structure, and can be replaced with heavy metal ions (Bailey et al., 1999; Sidheswaran and Bhat, 1997).
A wide variation in the cation-exchange capacity of zeolites was reported because of different nature of various cage structures of zeolites, natural structural defects, adsorbed ions and their associated gangue minerals (Mondale et al., 1995). Structural imperfections, a variety of dimensions, degree of hydration, and the presence of clays and other slime particles may lead to differences in properties between zeolites (Mondale et al., 1995). Reported sorption capacities of zeolites are (mg/g): Cd 84.3, Cr(III) 26.0, Hg 150.4, Pb 155.4 (Leppert, 1990). The overall sorption capacity was evaluated as ca. 1.5 meq/g.
In the present paper the ion exchange capacity and the uptake of mercury ions from model solutions and copper smelter and refinery effluent were studied with the use of the batch technique. The obtained results were confirmed in full-scale 7-days industrial tests in copper smelter and refinery wastewater treatment plant under ordinary technological regime.
2. Materials and methods
2.1. The effluent
The effluent was obtained from copper smelter and refinery-the large producer of electrolytic copper- cathodes (93,000 Mg/year) and round copper billets (35,000 Mg/year). The effluent production capacity is about 7000 m3/d. The company initially treated the effluent by sedimentation and pHadjustment, but this procedure was found to be ineffective in trace elements removal. The effluent has a pale yellow colour and elevated concentration of mercury ions.
2.2. Sorbent
Zeolite (finely grounded) was obtained from Sokornit mine ‘‘Druzba’’ (Ukraine) (the mining zeolite capacity of the ore is estimated as 126.1 million Mg and the annual production is 65,000–70,000 Mg) and was classified as clinoptilolite, which is high-silica member of heulandite group of natural zeolites and occurs in abundance and easily mined sedimentary deposits in many parts of the world. The unit cell formula of natural zeolites is (Li, Na, K)a (Mg, Ca, Sr, Ba)d[Alaþ2d Sin-ðaþsdÞO2n]mH2O (Abusafa and Yucel, 2002). The unit cell of clinoptilolite is usually characterized on the basis of 72 oxygen atoms (n ¼ 36) and 24 water molecules (m ¼ 24).
Zeolite used in this study contains 70% clinoptilolite, 9% silica and clays, 5% mica, 15% of zeolite water. The molar structure is (calculated as oxides): 0:20Na2O . 0:26K2O . 0:43CaO . 0:20MgO . 9:57ðSiO2 . Al2O3Þ. 0:09Fe2O3
2.3. Analytical methods
Mercury was analysed with Mercury analyser AMA 254 atomic absorption spectrometer (Czech Republic). pHwas measured with pH/ISE meter 920A with pH Orion Trioda 91–57 (USA) with 3-point calibration procedure. Adsorption surface area was determined with methylene blue method, described by Scheffler (1966).
2.4. Potentiometric titrations
Potentiometric titrations were performed in order to determine deprotonation constants and absolute concentrations of specific functional groups present on the sorbent surface. Zeolite (2 g) was suspended in deionised water (50 ml), through which argon had been bubbled for 3 h in order to remove dissolved CO2. The titration vessel was sealed immediately. Afterwards, zeolite was titrated with 0.1 M HCl/0.1 M NaOH. The suspension pHwas recorded after each addition of titrant.
2.5. Sorption experiments
Sorption isotherms were studied in model Hg2þ solutions (0.47 mg/kg) that corresponded to the concentration of mercury ions in the industrial effluent. Sorbent concentration was in the range 0.2–8.0 g/l. After the equilibrium was reached the suspension was filtered through membrane 0.2 lm millipore filter. The filtrate was analysed for the content of mercury ions.
3. Results and discussion
3.1. Mechanism of sorption with zeolite
In the literature there was found an inconsistence in evaluation of adsorption surface area of zeolites. Some authors report that these materials have surface of several hundreds m2/g, while the other report several m2/g. This inconsistency could be associated with differences in the properties of zeolites, their origin and the method of study. In the present work, adsorption surface area of the finely grounded zeolite (determined with methylene blue) was found to be rather low, 75 m2/g. This suggests that the material either has low sorption capacity or the dominating mechanism of sorption is different than physical adsorption.
Based on the chemical nature of zeolites, we assumed that the dominating mechanism of sorption was ion exchange in which cations bind to negatively charged groups on the sorbent surface. With the use of potentiometric titration with diluted acid, it was possible to determine deprotonation constants (pKa) and the surface concentration of each site capable of cation ex
change. Detailed chemical identification of each group was not possible. Literature reports that depending on pH, the zeolite surface should be charged, due to deprotonation of SiOHto SiO-groups while AlOH groups are protonated as AlOH2þ according to the corresponding shift of amphoteric equilibria (Parvulescu et al., 1995).
Fig. 1 shows a sample titration curve of Sokornit zeolite. Three inflexion points were observed, that correspond to three cation-exchange functional groups. The titration curve was described with three-protic acid model (Chojnacka, 2003). Inflexion points were determined from the first (reaches minimum) and the second (reaches zero) derivatives. Model parameters (pKa and concentration of each group) were determined with nonlinear regression (Mathematica ver. 3.0) (Table 1). Total protonated and thus available as binding sites for metal ions.
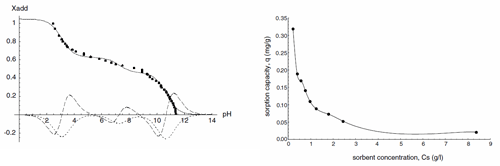
Fig. 1. Potentiometric titration curve of zeolite with 0.1 mol/l HCl modelled with polyprotic acid equation (–––) and its first (– – –) and second (–– ––) derivatives, (Xadd––the quantity of titrant added).
Fig. 3. Sorption capacity as the function of sorbent concentration.
Table 1 Functional groups capable of cation exchange present on the zeolite surface
| Acidic group | Acidic deprotonation constant, pKa |
Concentration of the group on the sorbent surface, (meq/g–) |
|---|---|---|
| 1 | 3.10 | 0.495 |
| 2 | 7.26 | 0.336 |
| 3 | 10.7 | 0.375 |
| ∑ 1.206 | ||
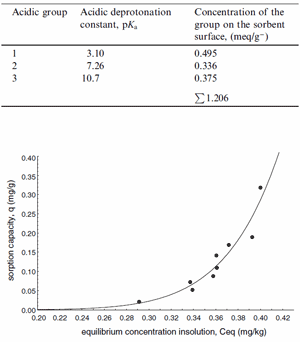
Fig. 2. Sorption isotherm for mercury removal with zeolite from diluted solutions; modelled with Langmuir–Freundlich equation (at temperature 21 °C).
3.2. Sorption kinetics and statics
The kinetic studies of sorption process with Sokornit zeolite revealed that sorption is first order reversible reaction (reaction constants kadsorption ¼ 3:36 1/h, kdesorption ¼ 4:81 1/h), the equilibrium was reached after 15 min. The results of equilibrium studies of mercury sorption on zeolite in diluted model solution (pH8.5) (with mercury concentration similar to the maximum observed concentration in the treated effluent) are shown in Fig. 2. Based on the experimental results (sorption studies and potentiometric titration), Langmuir–Freundlich model parameters (b; n) were determined with non-linear regression (Mathematica ver. 3.0). The maximum sorption capacity was 57.5 mg g -1, b ¼ 16:4, n ¼ 8:84. The presented isotherm described only equilibrium metal concentration and sorption capacity for diluted system, since the effluent that underwent treatment contained low level of mercury ions. The influence of sorbent concentration on sorption capacity is shown in Fig. 3.
3.3. Treatment of industrial effluent with zeolite – laboratory tests
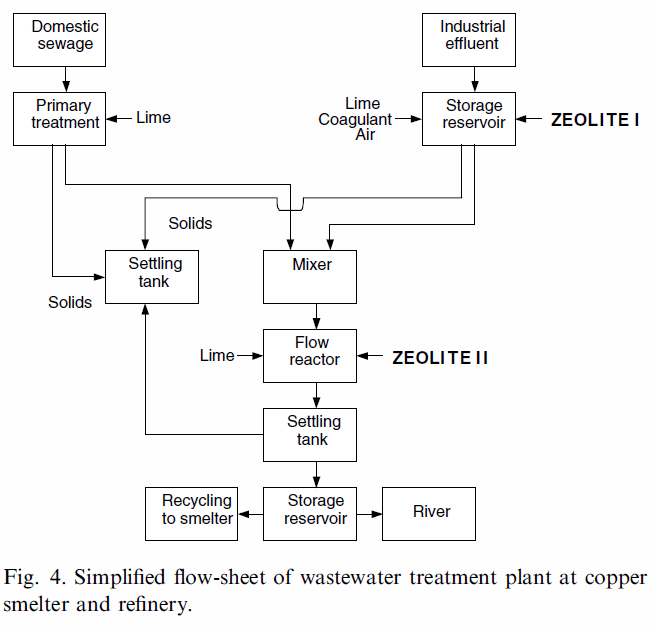
In order to choose the proper technological site of sorbent introduction (to reach the highest process efficiency), two effluents from copper smelter and refinery were studied: sewage (S, pH6.28), and mixed effluents: industrial and domestic (M, pH8.48) (Fig. 4), in which mercury content and pHwere analysed. Three different sorbent concentrations were applied (according to results obtained in the model experiments-Fig. 3): 0.35– 0.70–2.10 g/l.
The studies showed that the main source of mercury in the effluent was industrial wastewater stream. Low values of sorption capacity in these experiments could be explained with low concentration of mercury ions and competition of other cations that also undergo sorption (Table 2).
Table 2 Mercury removal from industrial effluents with Sokornit zeolite
| Sorbent concentration (g/l) |
Hg concentration in effluent S (mg/kg) |
Sorption capacity (mg/g) |
Hg concentration in effluent M (mg/kg) |
Sorption capacity (mg/g) |
|---|---|---|---|---|
| 0 | 0.0109 | 0.0246 | ||
| 0.35 | 0.0080 | 0.0083 | 0.0174 | 0.021 |
| 0.70 | 0.0062 | 0.0067 | 0.0143 | 0.015 |
| 2.10 | 0.0028 | 0.0039 | 0.0117 | 0.0061 |
3.4. Treatment of industrial effluent with zeolite – industrial tests
When considering the site of zeolite dosage, such factors as mercury concentration, the possibility of reaching the maximum sorption capacity and technological limitations of the installation should be considered. Two sites of zeolite introduction characterized with high turbulence conditions are marked in Fig. 4 as ‘‘Zeolite I’’ and ‘‘Zeolite II’’. The sorbent was dosed in two separate sites, in order to reach maximum removal efficiency. In the first place of zeolite introduction (Zeolite I site) the effluent contained the highest mercury concentration. The next site of dosage was after mixing with domestic sewage, containing lower concentrations of mercury ions (Zeolite II site). The doses applied were 75 kg/h that gave the average sorbent concentration 0.26 g/l. During the tests, mercury level was determined in the average daily samples that were sampled proportionally to the effluent flow rate. The results for mixed and treated effluents are shown in Fig. 5. After zeolite introduction stopped, ‘‘wash out’’ effect was observed due to decrease in the sorbent concentration in time. The effluent was sampled for the subsequent 48 h. This phenomenon was the evidence for mercury binding to zeolite, and was additionally confirmed by the rapid increase in metal concentration in the treated effluent until the level of the raw effluent was reached (Fig. 5).
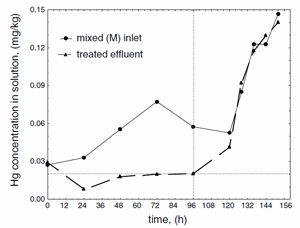
Fig. 5. Results of full-scale treatment of effluent with Sokornit zeolite; vertical line represents time at which zeolite dosage stopped; horizontal line represents Polish law requirements towards permissible levels of Hg2+.
4. Conclusion
Based on model experiments, it was found that Sokornit zeolite could be used for binding of cations in the process of ion exchange that was identified as the dominating mechanism of the process (beside physical adsorption). Due to the mechanism recognized as ionexchange, heavy metal ions sorption was found to be strongly pH-dependent. In this process, cations bind (under given pH) to deprotonated groups on zeolite surface. Three functional acidic groups were identified on the zeolite surface with pKa 3.10, 7.26 and 10.7. The maximum cation exchange capacity of zeolite was evaluated as 1.21 meq/g.
Mercury sorption with zeolite was found to be rapid, reversible, first order reaction. Equilibrium was reached after 15 min. Sorption isotherm was modelled with Langmuir–Freundlich equation based on sorption experiments carried out on dilute solutions (mercury concentration and pHwere similar to the industrial effluent).
Laboratory tests on mercury removal from the effluent from copper smelter and refinery showed the potential applicability of this method in the treatment that was confirmed by the full-scale tests. Mercury was removed below the level regulated by environmental pollution directives in streams of treated effluents that is 0.2 mg/kg. Due to the variability in the level of mercury in the effluent, it is necessary to perform further optimisation that would take into consideration installation aspects and configuration of the existing process.
References
- Abusafa, A., Yucel, H., 2002. Removal of 137Cs from aqueous solutions using different cationic forms of a natural zeolite: clinoptilolite. Separation and Purification Technology 28, 103–116.
- Bailey, S.E., Olin, T.J., Bricka, R.M., Adrian, D.D., 1999. A review of potentially low-cost sorbent for heavy metals. Water Research 33, 2469–2479.
- Chojnacka, K., 2003. Heavy metal ions removal by microalgae Spirulina sp. in the processes of biosorption and bioaccumulation. PhD Dissertation, Wroclaw University of Technology, Poland.
- Filippids, A., Godelitsas, A., Charistos, D., Misaelides, P., Kassoli- Fournaraki, A., 1996. The chemical behaviour of natural zeolites in aqueous environments: interactions between low-silica zeolites and 1 M NaCl solutions of different initial pH-values. Applied Clay Science 11, 199–209.
- Haidouti, C., 1997. Inactivation of mercury in contaminated soils using natural zeolites. Science of the Total Environment 208, 105– 109.
- Jurng, J., Lee, T.G., Lee, G.W., Lee, S.J., Kim, B.H., Seier, J., 2002. Mercury removal from incineration flue gas by organic and inorganic adsorbents. Chemosphere 47, 907–913.
- Leppert, D., 1990. Heavy metal sorption with clinoptilolite zeolite: alternatives for treating contaminated soil and water. Mining Engineering 42, 604–608.
- Ming, D.W., Dixon, J.B., 1987. Quantitative determination of clinoptilolite in soils by a cation-exchange capacity method. Clays and Clay Minerals 35, 463–468.
- Misaelides, P., Godelitsas, A., 1995. Removal of heavy metals from aqueous solutions using pretreated natural zeolitic materials: the case of mercury(II). Toxicological and Environmental Chemistry 51, 21–29.
- Mondale, K.D., Carland, R.M., Aplan, F.F., 1995. The comparative ion exchange capacities of natural sedimentary and synthetic zeolites. Mineral Engineering 8, 535–548.
- Morency, J.R., Panagiotou, T., Senior, C.L., 2002. Zeolite sorbent that effectively removes mercury from flue gases. Filtration and Separation 9, 24–26.
- Ouki, S.K., Kavannagh, M., 1997. Performance of natural zeolites for the treatment of mixed metal-contaminated effluents. Waste Management 15, 383–394.
- Panayotowa, M.I., 2003. Kinetics and thermodynamic of copper ions removal from wastewater by use of zeolite. Waste Management 23, 135–143.
- Parvulescu, V.I., Frunza, L., Catana, G., Russu, L., Parvulescu, V., 1995. Acidic ad textural properties of H-ZSM-5 impregnated with gallium, indium or thallium. Applied Catalysis A: General 121, 69– 79.
- Scheffler, G.H., 1966. Carbon activated. Snell-Ettra Encyclopaedia of Industrial Chemical Analysis 8, 139.
- Sidheswaran, P., Bhat, A.N., 1997. Impact of zeolitic water content on exchange of calcium ions. Thermochimica Acta 298, 55–58.
- Zamzow, M.J., Eichbaum, B.R., Sandgren, K.R., Shanks, D.E., 1990. Removal of heavy metals and other cations from wastewater using zeolites. Separation Science and Technology 25, 1555–1569.

