Evaluation of pathological changes in broilers during chronic aflatoxin (50 and 100 ppb) and clinoptilolite exposure
Abstract
This study was conducted to evaluate the pathological changes in broilers fed a diet containing low-levels of aflatoxin (AF) and clinoptilolite (CLI) until 42 days of age. A total of 576 one-day-old Ross-308 type broiler chicks were treated with varying levels of AF and CLI (15 gkg-1). The gross and histopathological changes in the liver, kidneys, spleen, thymus and bursa of Fabricius were investigated and relative organ weights were calculated. Compared to controls, significant changes (P < 0.05), such as slight to moderate hydropic degeneration and/or fatty change (8 cases of 10), bile-duct hyperplasia (7 of 10) and periportal fibrosis (5 of 10), were found in chicks fed 100 ppb AF-containing diet. No gross–pathological changes were observed in any treatments. The addition of CLI to the 100 ppb AF-containing diet significantly decreased the number of affected broilers and/or the severity of lesions (hydropic degeneration and bile-duct hyperplasia) in the livers (P < 0.05). The addition of CLI to the AF-free diet did not produce any significant lesions compared with the controls. © 2004 Published by Elsevier Ltd.
1. Introduction
Aflatoxins (AF), potent mycotoxins produced by Aspergillus flavus and Aspergillus parasiticus, are a major concern in the poultry production. The toxicity of AF in broiler chickens has been widely investigated by the determination of their carcinogenic, mutagenic, teratogenic (Wild et al., 2000; Sur and Celik, 2003) and growth inhibitory (Oguz and Kurtoglu, 2000) effects. The biochemical–haematological (Oguz et al., 2000a), immunological (Qureshi et al., 1998) and pathological (Dafalla et al., 1987; Kiran et al., 1998) toxic effects of AF have also been well described.
Contamination of AF in feed causes aflatoxicosis in poultry production characterised by listlessness, anorexia with lowered growth rate, poor feed utilisation, decreased weight gain, decreased egg weight and production, increased susceptibility to environmental and microbial stresses, and increased mortality (Leeson et al., 1995). AF can also cause important gross and microscopic changes in the liver, such as hepatomegaly, paleness, hydropic degeneration, fatty change, bile-duct hyperplasia and periportal fibrosis (Espada et al., 1992; Fernandez et al., 1994; Ledoux et al., 1999; Ortatatli and Oguz, 2001), kidney and spleen lesions (Glahn et al., 1991; Bilgic and Yesildere, 1992), unfavourable reproductive changes (Ortatatli et al., 2002), impairment of the humoral and cellular immune responses and increase susceptibility to some environmental and infectious agents (Ibrahim et al., 2000; Oguz et al., 2003).
Producers, researchers and governments aim to develop effective prevention management and decontamination technologies to minimise the toxic effects of AF. Besides of the preventive management, relatively new approaches have been employed including physical, chemical and biological treatments to detoxify AF in contaminated feeds and feedstuffs (Parlat et al., 2001). Since the beginning of 1990s, the adsorbent-based studies have been performed to remove AF from contaminated feed and minimise the toxicity of AF in poultry (Ibrahim et al., 2000). Zeolites (Miazzo et al., 2000), bentonites (Rosa et al., 2001) and clinoptilolite (CLI), which is a natural zeolite and a member of heulandite– stilbite group (Oguz and Kurtoglu, 2000; Oguz et al., 2000a,b), were preferred because of their high binding capacities for AF and their reducing effect on AF-absorption from the gastrointestinal tract.
The gross and histopathological changes in the target organs of AF in broilers have been widely investigated with higher AF levels in feed (1–5 ppm; mg kg -1) in the studies reviewed above. Also, the AF-detoxification studies have been employed with higher AF levels and in short-term periods. Therefore, the purpose of the present study was to evaluate the gross and microscopic changes in aflatoxicosis induced by low-levels (50 and 100 ppb) and chronic (42 days; one broiler production period) AF exposure, and to determine the possible preventive role of dietary CLI on the pathological changes examined.
2. Materials and methods
2.1. Chickens and diet
Five hundred and seventy-six one-day-old, Ross-308 type broiler chicks of both sexes were obtained from a commercial hatchery. Individually weighed chicks were divided at random into six groups. The chicks were housed in electrically heated compartments with continuous lighting and were fed a commercial feed starter (maize and soybean based, 230 g protein, 13.80 MJME kg -1) upto 21 days and thereafter a grower diet (215 g protein, 13.60 MJ ME kg -1) upto 42 days. Chickens were allowed access to the diets and water ad libitum. The starter and grower basal diets were both supplemented with amino acids, minerals and vitamins at levels recommended by National Research Council (NRC, 1994). The basal diets were tested for possible residual AF before feeding (Howel and Taylor, 1981), and there were no detectable levels present (detection limit: 1 lgkg -1 feed; recovery of the extraction method: 95%).
2.2. Experimental design
The experimental design consisted of six dietary treatments: (1) Control: Basal diet; (2) CLI: Basal diet + 15 g CLI kg -1 diet; (3) 50 ppb AF: Basal diet + 50 lg total aflatoxin (AF; the composition given below) kg -1 diet;
(4) 50 ppb AF + CLI: Basal diet + 50 lg AF+15 g CLI kg -1 diet; (5) 100 ppb AF: Basal diet + 100 lg AF kg -1 diet; (6) 100 ppb AF + CLI: Basal diet + 100 lg AF+15gCLIkg -1 diet. A commercially available CLI (KNa2Ca2(Si29AL7)O72 Æ 32H2O) was provided from the west region of Turkey (Incal Mining Ltd., Izmir, Turkey).
2.3. Aflatoxin
The AF was produced from A. parasiticus NRRL 2999 culture (USDA, Agricultural Research Service, Peoria, IL) via fermentation of rice by the method of Shotwell et al. (1966) with minor modifications by Oguz (1997). Successfully fermented rice was then steamed to kill the fungus, dried and ground to a fine powder. The AF content in rice powder was analysed by the method of Shotwell et al. (1966) and measured on a thin layer chromatography (TLC)–fluorometric densitometer (Ca-mag-III, Basel, Switzerland). The AF within the rice powder consisted of 72.51% AFB1, 14.05% AFB2, 9.78% AFG1 and 3.66% AFG2 based on total AF in the ground rice powder (detection limit: 1 lgAF kg -1 rice powder; recovery of the extraction method: 92%). The rice powder was added to the basal diet to provide the required amount of 50 and 100 ppb (lgkg -1 feed).
2.4. Pathological examination
When the chicks reached 42 days of age, the feeding trial was terminated and 10 broilers from each treatment were selected at random and killed for pathological examination. [Other chicks in the groups were used for the other parts of this project, in which the performance and immunological investigations were performed, and the findings were published previously (Oguz et al., 2000b; Oguz et al., 2003)]. Selected animals were weighed before being killed. A detailed necroscopy was then conducted. The liver, kidney, spleen, thymus and bursa of Fabricius were removed and weighed. The relative organ weights (weight of organ/100 g live body weights) were calculated. Tissue samples from these organs were collected in 10% neutral buffered formalin. After fixation, samples were dehydrated in alcohol, cleared in xylene and embedded in paraffin wax. Sections were cut at 5 lm and stained with haematoxylin and eosin (Thermo Shandon, 15275, USA). Some sections were also stained by the methods of van Gieson and Periodic Acid Shiff staining (Luna, 1968). Buffered formalin-fixed liver samples were sectioned by cryostat at 10–12 lm and stained for lipids using Sudan black staining method (Luna, 1968).
Microscopically, hepatocellular degeneration in livers was graded as follows; Slight (degree 1): Mild hepatocellular swelling due to hydropic degeneration and fatty changes only in centrilobular areas.
Moderate (degree 2): Clear hepatocellular swelling in both centrilobular and midzonal areas. Severe (degree 3): Diffuse and severe hepatocellular swelling, cytoplasmic paleness and rupture (this grade was not seen in any treatment).
2.5. Statistical analysis
The differences among the relative organ weights were analysed by Duncan’s multiple range test. The differences among the pathological changes were also determined by v 2 test (SPSS, 1999). Statements of statistical significance are based on a P < 0.05 significance level.
3. Results
The results, presented in Tables 1 and 2, show the effects of dietary treatments on relative weights and histopathological changes of organs. No statistical differences were found in relative weights of liver, kidney, spleen, thymus and bursa of Fabricius (Table 1). Also, no gross–pathological changes were observed in the treatment groups.
Microscopically, the livers in chickens fed diet containing 100 ppb AF showed significant lesions (P < 0.05), compared to control, such as slight to moderate hydropic degeneration and small fatty vacuoles in hepatocytes in centrilobular and midzonal areas (8 cases from 10; Fig. 1(a)). In the livers of some chickens in this group, a small amount of bile-duct proliferation located in portal areas (Fig. 2) and periportal fibrosis (Fig. 3), which was extending towards neighbouring portal triads, was also seen (7 and 5 cases, respectively; Table 2). The addition of 50 ppb AF to the diet caused very mild and statistically insignificant (P > 0.05) histopathological changes in livers in only 3 chicks, compared to controls. On the other hand, chicks fed 100 ppb AF also had lymphoid depletion in bursa of Fabricius (6 cases from 10; Fig. 4(a)) and slight tubular degeneration in the kidney (3 from 10). There were no considerable lesions in spleens and thymuses in the groups examined, except for very light lymphocytic depletion and cortical atrophy in a few cases.
Table 1 Effect of clinoptilolite (CLI; 15 g kg-1 diet) on relative organ weights for broiler chicks fed on a diet containing total aflatoxin (AF) between 1 and 42 days of age
| Treatment | Relative organ weights (organ weight /100 g live body weight) | ||||||
|---|---|---|---|---|---|---|---|
| AF (ppb) | Liver | Kidney | Spleen | Bursa of Fabricius | Thymus | ||
| 50 | 100 | CLI | |||||
| – | – | – | 2.283 ± 0.07 | 0.877 ± 0.03 | 0.112 ± 0.02 | 0.539 ± 0.04 | 0.260 ± 0.00 |
| – | – | + | 2.174 ± 0.06 | 0.837 ± 0.03 | 0.128 ± 0.01 | 0.500 ± 0.04 | 0.276 ± 0.02 |
| + | – | – | 2.349 ± 0.08 | 0.938 ± 0.04 | 0.119 ± 0.01 | 0.523 ± 0.06 | 0.264 ± 0.03 |
| + | – | + | 2.235 ± 0.09 | 0.830 ± 0.05 | 0.117 ± 0.01 | 0.521 ± 0.04 | 0.308 ± 0.02 |
| – | + | – | 2.268 ± 0.06 | 0.932 ± 0.04 | 0.128 ± 0.01 | 0.599 ± 0.05 | 0.284 ± 0.03 |
| – | + | + | 2.264 ± 0.08 | 0.832 ± 0.04 | 0.135 ± 0.01 | 0.508 ± 0.04 | 0.288 ± 0.02 |
Values represent the mean ± SEM of six groups of 10 broiler chicks each per treatment.
No statistically differences were found within the treatments according to the Duncan’s multiple range tests.
Table 2 Effect of clinoptilolite (CLI; 15 g kg-1 diet) on gross and microscopic changes for broiler chicks fed on a diet containing total aflatoxin (AF) between 1 and 42 days of age
| Changes/Lesions | Group 1 (Control) |
Group 2 (CLI) |
Group 3 (50 ppb AF) |
Group 4 (50 ppb AF + CLI) |
Group 5 (100 ppb AF) |
Group 6 (100 ppb AF + CLI) |
|---|---|---|---|---|---|---|
| LIVER | 1/10b | 2/10b | 3/10b | 2/10b | 8/10a | 5/10ab |
| Hydropic degeneration and/or fatty changesa | ||||||
| Slight | 1/10a | 2/10a | 3/10a | 2/10a | 4/10a | 5/10a |
| Moderate | –/10b | –/10b | –/10b | –/10b | 4/10a | –/10b |
| Bile-duct proliferation | –/10b | 3/10ab | 2/10b | 2/10b | 7/10a | 2/10b |
| Periportal fibrosis | 1/10ab | –/10b | 1/10ab | –/10b | 5/10a | 2/10ab |
| Tubular degeneration inKIDNEY | 1/10a | 1/10a | 2/10a | 1/10a | 3/10a | 2/10a |
| Lymphoid depletion inSPLEEN | –/10b | –/10b | 1/10a | 1/10a | 2/10a | 1/10a |
| Cortical atrophy in THYMUS | –/10b | 1/10a | 1/10a | –/10a | 2/10a | 1/10a |
| Lymphoid depletion in BURSA of FABRICIUS | 2/10a | 2/10a | 3/10a | 2/10a | 6/10a | 3/10a |
The values represent the number of chicks showing changes/number of chicks examined in each treatment group.
Values within lines with no common superscripts are significantly different (P < 0.05), according to the v2 tests.
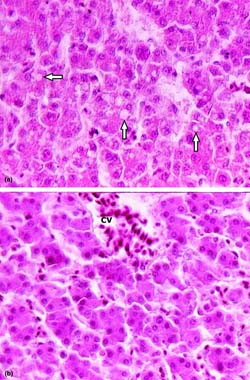 Fig. 1. Comparative microscopic appearance of livers. (a) 100 ppb AF-treated group. Moderate hydropic degeneration and vacuoles in hepatocytes (arrows), (b) 100 ppb AF plus CLI group. Normal aspect of unaffected hepatocytes from AF exposure and a hyperemic central vein (cv). H & E × 650.
Fig. 1. Comparative microscopic appearance of livers. (a) 100 ppb AF-treated group. Moderate hydropic degeneration and vacuoles in hepatocytes (arrows), (b) 100 ppb AF plus CLI group. Normal aspect of unaffected hepatocytes from AF exposure and a hyperemic central vein (cv). H & E × 650.
The addition of CLI (15 g kg -1) to 100 ppb AF-containing diet (Group 6) partially decreased both the incidence of affected broilers and the severity of lesions in the organs investigated in this study (Table 2, Figs. 1(b), 4(b)). Especially, the improvements in the severity of hydropic degeneration and in the bile-duct hyperplasia in livers were significant (P < 0.05). The addition of CLI to the AF-free diet (Group 2) did not cause any significant gross and microscopic changes in chicks when compared with the control birds (Table 2).
4. Discussion
The gross and microscopic investigations in the previous studies showed that AF affected the organs belonging to the haematopoietic, immune and reticulo-endothelial systems (Dafalla et al., 1987; Qureshi et al., 1998; Ortatatli and Oguz, 2001). In this study, the toxic effects of AF and the ameliorative efficacy of dietary adsorbent (CLI) on the detrimental effects of AF were investigated in the point of pathological to be target organs for AF and these AF levels that affect broilers in terms of performance, are primarily affected in aflatoxicosis cases. The partic-haematology, enzyme biochemistry, immunology and ular aim of this study was to induce chronic aflatoxi-histology. In this study, the toxic effects of AF on liver, cosis in broilers during one broiler production period kidney and bursa of Fabricius have been clearly observed (42 days) with lower AF levels (50 and 100 ppb), by feeding 100 ppb AF fed for 42 days (Table 2; Figs. 1(a), which may naturally occur under field conditions. 2, 3 and 4(a)). However, it cannot be concluded from the present investigation that whether 50 ppb AF level causes effects of AF on the target organs with higher levels of AF aflatoxicosis in broilers as no significant difference was observed compared to the control group (P > 0.05). This critical AF level (100 ppb) has been supported by other studies that reported the clinical, haematological– biochemical and histopathological changes began at 100 ppb onwards in feed in broilers (Giambrone et al., 1985; Marquez and Hernandez, 1995; Khajarern and Khajarern, 1999). The published report on performance of the same broilers used in this study showed that 100 ppb AF in feed significantly reduced the performance of chicks (Oguz et al., 2000b) in agreement with the pathological results in the present study. However, the humoral immunity of these chicks was affected in the lower AF dose (50 ppb) in feed (Oguz et al., 2003).
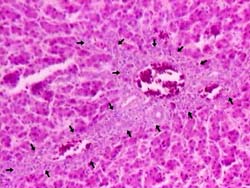
Fig. 2. Liver from 100 ppb AF-treated group Bile-duct proliferation (arrows) and mononuclear cells infiltration in the portal triad and mild hydropic degeneration of hepatocytes. H & E × 260.
That no macroscopic changes were found even in target organs in this study could be due to the lower levels of AF used. However, even if mild, some histopathological changes (Table 2) were seen in liver, kidneys and bursa Fabricii from the chicks receiving the 100 ppb AF-alone diet. This observation suggests that a reliable evaluation could not be performed by macroscopic investigations of organs alone and that histopathological examination was also important. At the same time, the moderate histopathological changes observed in this study are in agreement with the results of previous studies performed by various lower levels of AF (100–500 ppb) in broilers (Giambrone et al., 1985; Marquez and Hernandez, 1995), laying hens (Dafalla et al., 1987), ducks (Sell et al., 1998; Khajarern and Khajarern, 1999) and wild turkeys (Quist et al., 2000). Previous studies have stated that the periportal fibrosis and bile-duct hyperplasia findings, in particular, may constitute chronic aflatoxicosis cases and indicate the regenerative changes in the liver. Espada et al. (1992) have also reported that vacuolation of liver cells and cellular depletion in the follicle medulla of the bursa Fabricii appeared first and persisted during the recovery phase in experimental aflatoxicosis. These findings support our results, indicating that chronic aflatoxicosis can be produced by feeding lower levels of AF (100 ppb) over a long-term period (42 day, one broiler production period).
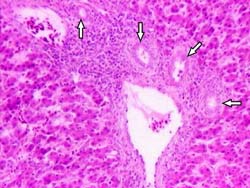
Fig. 3. Liver from 100 ppb AF-treated group. Periportal fibrosis extending towards neighbouring portal triad and into the lobulus (surrounded with arrows) H & E × 260.
The second aim of this study was to evaluate the possible preventive effect of CLI, which was simultaneously added to the broiler feed for 42 days. The addition of CLI (15 g kg -1) to the AF-containing diet (group 6) partially reduced the severity of lesions in the organs examined (Table 2, Figs. 1(b) and 4(b)). The beneficial effect of CLI in this study might be attributed to act as a sequestering agent against AF in feeds through its adsorption and thereby reducing the AF bioavailability in the gastrointestinal tract, such as other zeolites (Harvey et al., 1993; Miazzo et al., 2000). The CLI–AF interaction involves the formation of complex by the B-carbonyl system of the AF with free radicals of the adsorbents (Bailey et al., 1998). Previous studies have reported that the addition of CLI (15 gkg -1) to the high AF (2.5 ppm)-containing diet significantly reduced the growth inhibitory effects of AF (Oguz and Kurtoglu, 2000) and biochemical–haematological toxic effects (Oguz et al., 2000a) in broiler chicks fed for 21 days.
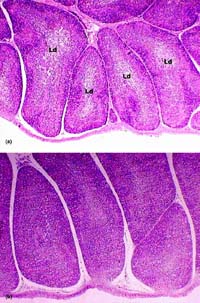
Fig. 4. Bursa of Fabricius. (a) 100 ppb AF-treated group. Moderate lymphoid depletion (Ld) in the centre of follicles, (b) 100 ppb AF plus CLI group. Unaffected normal follicles. H & E × 65.
In the long-term trials, which were the other parts of this study, an intermediate amelioration was observed in the adverse effects of low AF levels (100 ppb) on growth inhibitory (Oguz et al., 2000b) and immunotoxic effects (Oguz et al., 2003) of AF in broilers by dietary CLI (15 g kg -1). CLI also provided significant improvements on performances of Japanese quail by the addition of CLI to the AF (2 ppm)-containing diet for 35 days (Parlat et al., 1999). However, Harvey et al. (1993) found no beneficial effect on broiler chicks by adding CLI (5 g kg -1) to an AF (3.5 ppm)-containing diet for 21 days. The reason for these differences may be due to the different type, dose, and physical characteristics of CLI, concentration of AF in the diet or the broiler strain used in the trials. CLI has also been known as an inert and non-toxic matter for poultry (Olver, 1997; Oguz and Kurtoglu, 2000). Our findings have supported these reports, because the addition of CLI (15 g kg -1) to the AF-free diet (group 2) did not cause any adverse effects on investigated parameters in this study (Table 2) as indicated by Ortatatli and Oguz (2001).
These results clearly demonstrated that slight and moderate histological lesions were observed in chickens fed a diet containing 100 ppb AF while no macroscopically lesions were seen and that the simultaneous addition of CLI to the AF-containing diet provided a moderate amelioration in AF toxicity. Furthermore, CLI was inert and non-toxic for broilers. These improvements should contribute to a solution of the AF problem in broiler chickens, when used with other mycotoxin management practices.
References
- Bailey, R.H., Kubena, L.F., Harvey, R.B., Buckley, S.A., Rottinghaus, G.E., 1998. Efficacy of various inorganic sorbents to reduce the toxicity of aflatoxin and T-2 toxin in broiler chickens. Poultry Science 77, 1630–1632.
- Bilgic, H.N., Yesildere, T., 1992. Renal lesions on experimental aflatoxicosis in chickens. I.U. Veteriner Fakultesi Dergisi 18, 102–108.
- Dafalla, R., Yagi, A.I., Adam, S.E.I., 1987. Experimental aflatoxicosis in hybro-type chicks; sequential changes in growth and serum constituents and histopathological changes. Veterinary and Human Toxicology 29, 222–225.
- Espada, Y., Domingo, M., Gomez, J., Calvo, M.A., 1992. Pathological lesions following an experimental intoxication with aflatoxin B1 in broiler chickens. Research in Veterinary Science 53, 275–279.
- Fernandez, A., Verde, M., Gascon, M., Ramos, J., Gomez, J., Luco, D.F., Chavez, G., 1994. Variations of clinical, biochemical parameters of laying hens and broiler chickens fed aflatoxincontaining feed. Avian Pathology 23, 37–47.
- Giambrone, J.J., Diener, U.L., Davis, N.D., Panangala, V.S., Hoerr, F.J., 1985. Effects of aflatoxin on young turkeys and broilers chickens. Poultry Science 64, 1678–1684.
- Glahn, R.P., Beers, K.W., Bottje, W.G., Wideman, R.F., Huff, W.E., Thomas, W., 1991. Aflatoxicosis alters avian renal function, calcium, and vitamin D metabolism. Journal of Toxicology and Environmental Health 34, 309–321.
- Harvey, R.B., Kubena, L.F., Ellisalde, M.H., Phillips, T.D., 1993. Efficacy of zeolitic ore compounds on the toxicity of aflatoxin to growing broiler chickens. Avian Diseases 37, 67–73.
- Howel, M.V., Taylor, P.W., 1981. Determination of aflatoxins, ochratoxin A, and zearalenone in mixed feeds, with detection by thin layer chromatography or high performance liquid chromatography. Journal of the Association of Official Analytical Chemistry 64, 1356–1363.
- Ibrahim, I.K., Shareef, A.M., Al-Joubory, K.M.T., 2000. Ameliorative effects of sodium bentonite on phagocytosis and Newcastle disease antibody formation in broiler chickens during aflatoxicosis. Research in Veterinary Science 69, 119–122.
- Khajarern, J., Khajarern, S., 1999. Positive effects of Mycosorb against aflatoxicosis in ducklings and broilers. In: Poster presentation at Alltech’s 15th Annual Symposium on Biotechnology in the Feed Industry, Lexington, KY.
- Kiran, M.M., Demet, O., Ortatatli, M., Oguz, H., 1998. The preventive effect of polyvinyl–polypyrrolidone on aflatoxicosis in broilers. Avian Pathology 27, 250–255.
- Ledoux, D.R., Rottinghaus, G.E., Bermudez, A.J., Alanso-Debolt, M., 1999. Efficacy of hydrated sodium calcium aluminosilicate to ameliorate the toxic effects of aflatoxin in broiler chicks. Poultry Science 78, 204–210.
- Leeson, S., Diaz, G., Summers, J.D., 1995. Aflatoxins. In: Leeson, S., Diaz, G., Summers, J.D. (Eds.), Poultry Metabolic Disorders and Mycotoxins. University Books, Canada, Ont., pp. 248–279.
- M. Ortatatli et al. / Research in Veterinary Science 78 (2005) 61–68
- Luna, L.G., 1968. Manuel of Histologic Staining Methods of the Armed Forces Institute of Pathology, third ed. McGraw-Hill, New York.
- Marquez, R.N.M., Hernandez, T.R., 1995. Aflatoxin adsorbent capacity of two Mexican aluminosilicates in experimentally contaminated chick diet. Food Additives and Contaminants 12, 431–433.
- Miazzo, R., Rosa, C.A., De Queiroz Carvalho, E.C., Magnoli, C., Chiacchiera, S.M., Palacio, G., Saenz, M., Kikot, A., Basaldella, E., Dalcero, A., 2000. Efficacy of synthetic zeolite to reduce the toxicity of aflatoxin in broiler chicks. Poultry Science 79, 1–6.
- National Research Council, 1994. Nutrient Requirements of Poultry, 9th ed., National Academy Press, Washington, DC, pp. 44– 45.
- Oguz, H., 1997. The preventive efficacy of polyvinylpolypyrrolidone (PVPP) alone and its combination with the other adsorbents into broiler feeds against aflatoxicosis. Ph. D. Thesis. Institute of Health Sciences, University of Selc¸uk, Konya.
- Oguz, H., Kurtoglu, V., 2000. Effect of clinoptilolite on fattening performance of broiler chickens during experimental aflatoxicosis. British Poultry Science 41, 512–517.
- Oguz, H., Kecec, T., Birdane, Y.O., Onder, F., Kurtoglu, V., 2000a. Effect of clinoptilolite on serum biochemical and haematological characters of broiler chickens during experimental aflatoxicosis. Research in Veterinary Science 69, 89–93.
- Oguz, H., Kurtoglu, V., Coskun, B., 2000b. Preventive efficacy of clinoptilolite in broilers during chronic aflatoxin (50 and 100 ppb) exposure. Research in Veterinary Science 69, 197–201.
- Oguz, H., Hadimli, H.H., Kurtoglu, V., Erganis, O., 2003. Evaluation of humoral immunity of broilers during chronic aflatoxin (50 and 100 ppb) and clinoptilolite exposure. Revue de Medicine Veterinaire 154, 483–486.
- Olver, M.D., 1997. Effect of feeding clinoptilolite (zeolite) on the performance of three strains of laying hens. British Poultry Science 38, 220–222.
- Ortatatli, M., Oguz, H., 2001. Ameliorative effects of dietary clinoptilolite on pathological changes in broiler chickens during aflatoxicosis. Research in Veterinary Science 71, 59–66.
- Ortatatli, M., Ciftci, M.K., Tuzcu, M., Kaya, A., 2002. The effects of aflatoxin on the reproductive system of roosters. Research in Veterinary Science 72, 29–36.
- Parlat, S.S., Yildiz, A.O., Oguz, H., 1999. Effect of clinoptilolite on fattening performance of Japanese quail (Coturnix coturnix japonica) during experimental aflatoxicosis. British Poultry Science 40, 495–500.
- Parlat, S.S., Ozcan, M., Oguz, H., 2001. Biological suppression of aflatoxicosis in Japanese quail (Coturnix coturnix japonica)by dietary addition of yeast (Saccharomyces cerevisiae). Research in Veterinary Science 71, 207–211.
- Quist, C.F., Bounous, D.I., Kilburn, J.V., Nettles, V.F., Wyatt, R.D., 2000. The effect of dietary aflatoxin on wild turkey poults. Journal of Wildlife Disease 36, 236–444.
- Qureshi, M.A., Brake, J., Hamilton, P.B., Hagler, W.M., Nesheim, S., 1998. Dietary exposure of broiler breeders to aflatoxin results in immune dysfunction in progeny chicks. Poultry Science 77, 812–819.
- Rosa, C.A., Miazzo, R., Magnoli, C., Salvano, M., Chiac, S.M., Ferrero, S., Saenz, M., Carvalho, E.C., Dalcero, A., 2001. Evaluation of the efficacy of bentonite from the south of Argentina to ameliorate the toxic effects of aflatoxin in broilers. Poultry Science 80, 139–144.
- Sell, S., Xu, K.L., Huff, W.E., Kubena, L.F., Harvey, R.B., Dunsford, H.A., 1998. Aflatoxin exposure produces serum a fetoprotein elevations and marked oval cell proliferation in young male Pekin ducklings. Pathology 30, 34–39.
- Shotwell, O.L., Hesseltine, C.V., Stubblefield, R.D., Sorenson, W.G., 1966. Production of aflatoxin on rice. Applied Microbiology 14, 425–429.
- SPSS 1999. SPSS/PC + V.10.0. Base Manuel for the IBM PC/XT/AT and PS/2. Marija and Morusis, SPSS Inc.
- Sur, E., Celik, I., 2003. Effects of aflatoxin B1 on the development of bursa of Fabricius and blood lymphocyte acid phosphatase of the chicken. British Poultry Science 44, 558–566.
- Wild, C.P., Yin, F., Turner, P.C., Chemin, I., Chapot, B., Mendy, M., Whittle, H., Kirk, G.D., Hall, A.J., 2000. Environmental and genetic determinants of aflatoxin–albumin adducts in the Gambia. International Journal of Cancer 86, 1–8.

